Flow-Through Cell-Counting Assay (FTCA)
Individuals with HIV infection and AIDS exhibit abnormalities of the immune system, reflected largely in their CD4+ T lymphocytes, which are targeted by the virus. In most HIV-infected persons there is a gradual decline in CD4 cells, whereas people who are successfully treated demonstrate an increase in CD4 lymphocytes. Measurement of CD4 lymphocyte levels provides information that guides therapy and predicts disease outcome. In general, CD4 counts should be monitored every 3-6 months in HIV-infected individuals in order to: 1) determine when to start Highly Active Antiretroviral Therapy (HAART), 2) evaluate response to HAART, and 3) assess the need for initiation or discontinuation of prophylaxis for opportunistic infections [1].
The cost of CD4 testing is a critical barrier in managing the global HIV pandemic. There are several CD4 technologies on the market however, the cost, feasibility, ease of use, and performance characteristics of these technologies vary extensively. The current gold standard technology for measuring CD4 counts in whole blood is the fluorescent activated cell count and sorting systems (FACS) [2, 3]. Tests based on FACS are costly and highly complex, which usually means that these tests are not accessible in the resource limited settings, such as developing countries. HAART has been available in developing countries for more than a decade and free of charge through philanthropic resources such as the Bill and Melinda Gates Foundation, the Clinton Health Access Initiative, and governmental resources such as the President’s Emergency Fund for AIDS Relief (PEPFAR) [4]. However, the expense of CD4 testing remains a problem, and as a result, many patients are missing the appropriately timed initiation of HAART treatment [5]. Individuals who start HAART late have poorer outcomes including quicker disease progression and increased mortality when compared to individuals who start HAART early [6]. The World Health Organization (WHO) has pointed out or recommended that a handheld, point-of-care, reliable, and affordable CD4 count platform is urgently needed in resource-limited settings [7].
ALLIS is developing a Flow-Through Counting Assay (FTCA) which has the potential to meet WHO criteria for point of care tests and has several advantages over other manual rapid tests under development and on the market. Advantages of FTCA include it’s extreme simplicity and low cost per test. Our Flow-Through Cell Counting Assay offers consistent semi-quantitative results that can be utilized to determine when to start HAART in HIV patients, even in resource-limited settings. While a number of manual point-of-care assays have emerged to meet demands for low-cost rapid testing of CD4 cell counts, significant limitations prevent widespread adoption of these assays, such as multiple step procedures, potential for error and lengthy turnover times for obtaining results. FTCA produces test results in less than 30 minutes and can be performed by laboratory personnel with low levels of training. FTCA makes clever use of commoditized glucose meter technology for reading out rapid, semiquantitative results. This will allow rapid global deployment of tests via partnership with existing diagnostics manufacturers. Alternatively, absolute cell counts may be made using imaging devices, if desired.
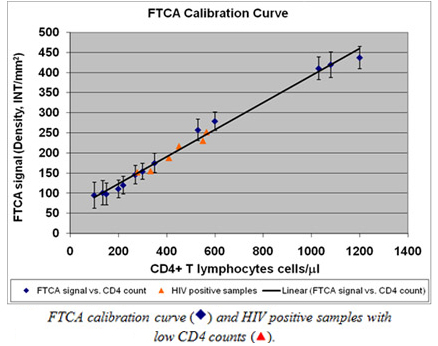
FTCA is based on the use of a special membrane that selectively captures white blood cells (WBC) and a proprietary flow-through cassette to measure cell count status. The FTCA calibration curve is shown in Figure 1. As can be seen in this Figure, the calibration curve is linear in the whole clinically significant range of CD4 T-cells concentrations. The most important feature of the FTCA calibration curve is that it allows accurate measurement in the region of CD4 T-cell counts equal to 350 cells/ml, the cut-off CD4 concentration used for making the decision for using HAART treatment for HIV positive patients.
Critical advantages of the FTCA are
- Semi-quantitative or quantitative CD4 counts in the clinically significant range of CD4 concentrations;
- Short time of analysis;
- Low cost;
- Simplicity.
Related Patents and Publications
Patents
Bystryak S, Santockyte R. A flow through cell counting assay.
(A) US Patent No. 9,097,712 (2015).
(B) European Patent No. EP2972345B1 (2018).
Publications
- Bystryak S, Bandwar RP, Santockyte R. A flow-through cell counting assay for point-of-care enumeration of CD4 T-cells. J. Virol. Methods, 2019, 271, 113672. Link
- Bystryak S, Acharya C, Dobiszewski K, Zhu H, Bandwar RP. Preclinical Assessment of a Cartridge-Based Flow-Through Assay for Determination of Adult CD4 T-Cell Count. Open AIDS J., 2020, 14(1), 50-60. Link
References
- Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, 2011: p. 1-66.
- WHO: CD4+ T-Cell Enumeration Technologies; Technical Information. GENEVA: World Health Organization (WHO), 2008.
- Yager P, Domingo GJ, Gerdes J: Point-of-care diagnostics for global health. Annu Rev Biomed Eng. 2008;10: 107-44.
- Chandrasekaran P, Dallabetta G, Loo V, Mills S, Saidel T, Adhikary R, Alary M, Lowndes CM, Boily MC, Moore J: Evaluation design for large-scale HIV prevention programmes: the case of Avahan, the India AIDS initiative. AIDS. 2008; 22 Suppl 5: S1-15.Boyle DS, Hawkins KR, Steele MS, Singhal M, Cheng X: Emerging technologies for point-of-care CD4 T-lymphocyte counting. Trends Biotechnol. 2012; 30(1):45-54.
- Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents – November 2009.
- Moon S, Gurkan UA, Blander J, Fawzi WW, Aboud S, Mugusi F, Kuritzkes DR, Demirci U: Enumeration of CD4 T-Cells Using a Portable Microchip Count Platform in Tanzanian HIV-Infected Patients. PLoS One. 2011; 6: e21409.
