AmpliFlux Technology
The industry standard technology Enzyme-Linked Immuno-Sorbent Assay (ELISA) is widely used in biology and medicine for the detection of viruses, bacteria, disease biomarkers, and other physiologically active substances. It is used routinely in research, clinical and pharmaceutical laboratories. However, in many cases the sensitivity of ELISA is inadequate. Thus, increasing the sensitivity, accuracy and general performance of these assays offers a significant opportunity to address unmet clinical needs. Our AmpliFlux technology improves the sensitivity and accuracy of numerous diagnostic assays.
Based on the combination of its patented and proprietary technologies, ALLIS has developed the AmpliFlux method for drastically increasing the sensitivity of ELISA-based assays.In the most commonly used ELISA, the “sandwich ELISA”, capture antibodies for the analyte are attached to a solid phase, typically a polystyrene microtiter plate. A liquid sample with unknown concentration of the analyte and an enzyme-labeled second antibody to the analyte are added to wells in the plate. As a result, an antibody-analyte-enzyme-labeled antibody complex is formed on the surface of the solid phase. After incubation, unbound analyte and enzyme-labeled antibody are removed from the solution by washing. Next, a substrate solution is added to the well and the amount of the bound enzyme-conjugated antibody is determined by detecting color, fluorescence or luminescence signals in the final reaction mixture. The amount of the captured enzyme-linked antibody on the surface of the microtiter plate is proportional to the concentration of the analyte. Therefore, the analyte concentration can be calculated from a calibration curve prepared using solutions with known analyte concentration (standards).
AmpliFlux is based on the use of a unique photochemical amplification reaction and consists of two steps: the first step is a conventional ELISA. In the second step, AmpliFlux Amplification Reagent is added to the well instead of the kit substrate solution and the mixture is irradiated with visible light. Illumination of the samples leads to a drastic increase in the final signal-to-noise ratio. This step typically takes several minutes.
Example. Determination of HIV-1 p24 antigen by the AmpliFlux Method
To demonstrate the AmpliFlux method, we show results for the detection of HIV-1 p24 using reagents from the Alliance HIV-1 P24 ELISA kit (Perkin Elmer Life Sciences, (Boston, MA). The Alliance HIV-1 P24 ELISA kit is used to carry out p24 quantification assays in two formats: the immune complex disruption (ICD) and non-ICD. In the ICD assay format, the immobilized monoclonal antibody captures both free HIV-1 p24 and p24 that has been released upon disruption of immune complexes in serum or plasma. In the non-ICD format, serum/plasma samples do not undergo immune complex disruption. The non-ICD assay format is used for detecting early infection during the earlier part of the window period due to its high analytical sensitivity, whereas the ICD assay format is used to detect cases during the later part of the window period.
The HIV-1 P24 ELISA kit contains a 96-well microtiter plate with wells coated with a highly specific mouse monoclonal antibody to HIV-1 p24. The captured antigen is complexed with biotinylated polyclonal antibody to HIV-1 p24 and streptavidin-HRP (horseradish peroxidase) conjugate. The concentration of the analyte is determined by measuring the signal (optical density) of the solution in the microplate well and calculating the concentration from a calibration curve generated by standards run on the same plate. The detection limit of the tests was estimated as the analyte concentration corresponding to twice the value of the background signal.
Results: The calibration curves for the determination of HIV-1 p24 antigen using non-ICD assay format for the conventional ELISA and ELISA + AmpliFlux are shown in Figure 1. Figures 1A and 1B show the results obtained in low and high range of HIV-1 p24 antigen concentrations, respectively. The results summarized in Table 1 show that the limit of detection of HIV-1 p24 for the conventional non-ICD assay is 3.5 pg/ml, whereas the detection limit for the non-ICD ELISA + AmpliFlux is approximately 0.08 pg/ml at 12 min of illumination (Table 1). Thus, the analytical sensitivity of the assay increases more than 40-fold using AmpliFlux.
Similar results for the determination of HIV-1 p24 antigen were obtained using ICD assay format (Table 1).
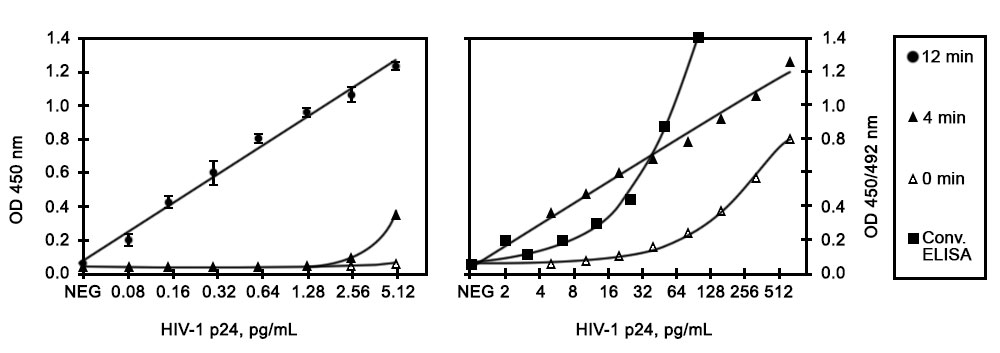
Figure 1. Calibration curves for the determination of HIV-1 p24 antigen using conventional non-ICD ELISA (B, squares) and ELISA + AmpliFlux at low (A) and high range (B) of HIV p24 antigen concentrations.
| Table 1. Sensitivity of conventional ELISA and ELISA + Ampliflux HIV-1 p24 assays | ||||
| Non-ICD | ICD | |||
| Method | Analytical sensitivity, pg/mL | Increase in sensitivity | Analytical sensitivity, pg/mL | Increase in sensitivity |
| Conventional ELISA | 3.5 | 1 | 4 | 1 |
| ELISA + AmpliFlux | 0.08 | 44 | 0.1 | 40 |
Conclusion: AmpliFlux technology allows increased sensitivity, accuracy, dynamic range, and signal-to-noise ratio of ELISA assays, while saving reagent costs and reducing time of analysis. Outstanding results have been achieved for most other analytes tested including Hepatitis B, Prostate specific antigen (PSA), amyloid Beta (1-42), Phospho-tau (Thr217) and other physiologically active substances.
Fluorescence Enhanced Technique (FET)
In addition to the AmpliFlux technology, ALLIS has developed an alternative method of enhancing the sensitivity of commercially available ELISA assays, FET (Fluorescence Enhanced Technique). In FET, after performing conventional ELISA an enhancement reagent is added to the substrate solution and the fluorescence of the sample is measured. Results obtained using FET for HIV-1 p24 assay are presented in Figure 2.
Typically, the analytical sensitivity of ELISA + FET is 5-10-fold higher than that for the corresponding conventional ELISA.
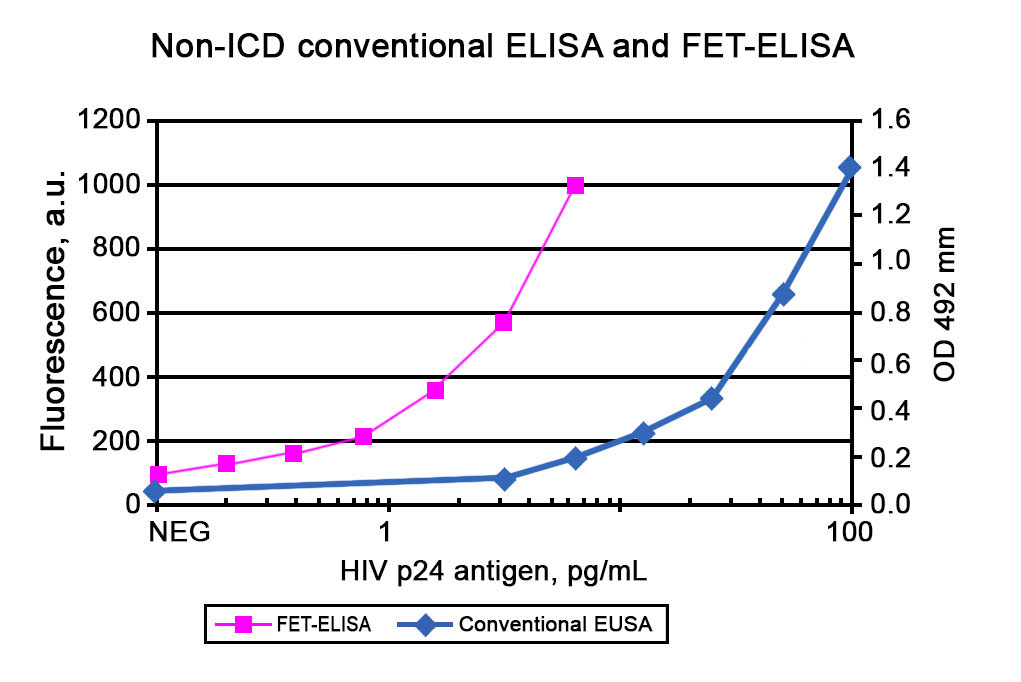
Figure 2. Calibration curves for determination of HIV-1 p24 antigen using non-ICD using conventional ELISA and ELISA + FET
Related Patents and Publications
Patents
S. Bystryak, R. Santockyte. METHODS FOR IMPROVING ANALYTE DETECTION USING PHOTOCHEMICAL REACTIONS. US Patent 8,916,341.
S. Bystryak, R. Santockyte. METHODS FOR IMPROVING ANALYTE DETECTION USING PHOTOCHEMICAL REACTIONS. US Patent 8,951,722
Bystryak S, Santockyte R. METHODS FOR IMPROVING ANALYTE DETECTION USING PHOTOCHEMICAL REACTIONS. European patent EP3066471
Publications
- S. Bystryak, R. Santockyte. Increased Sensitivity of HIV-1 p24 ELISA Using a Photochemical Signal Amplification System. J Acquir Immune Defic Syndr, 2015, 70 (2), 109-114. link
- S. Bystryak, C. Acharya. Detection of HIV-1 p24 antigen in patients with varying degrees of viremia using an ELISA with a photochemical signal amplification system. Clinica Chimica Acta, 2016, 456, 128-136. link
